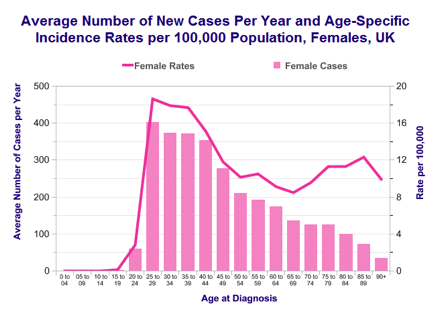
- 3,000 new cases per year in UK
- 9 cases per 100,000 women per year in UK
- Life-time risk of 1 in 135
- Most common cancer in women under 35 years of age
- 7 out of 10 cases are Squamous Cell Cancer type
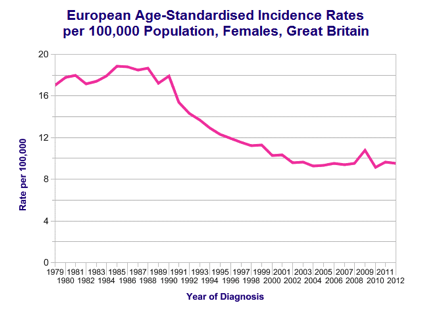
- Incidence rates have reduced significantly since the introduction of screening programme in 1988
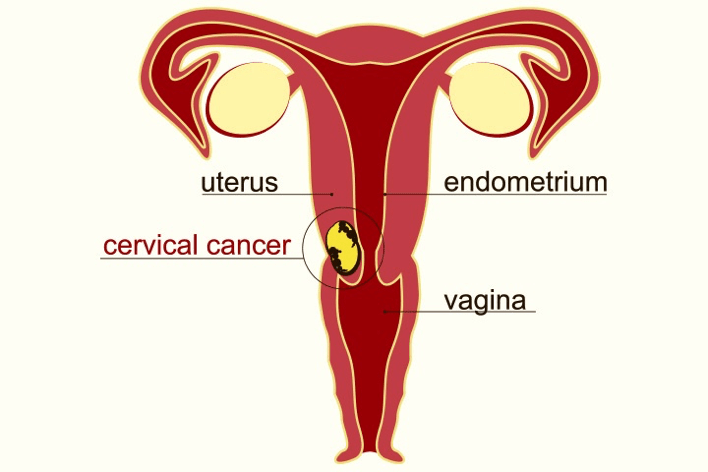
Risk factors and Causes
- Age
- Human papillomavirus (HPV)
- Human immunodeficiency virus (HIV)
- Sexually transmitted infections (STIs)
- Previous vulval, vaginal, anal or oro-pharyngeal cancer
- Untreated high-grade cervical intraepithelial neoplasia (VIN)
- Not taking part in cervical screening programme
- Sexual and reproductive activity (Early age of onset, number of partners, number of pregnancies and age at first pregnancy)
- Auto-immune conditions such as systemic lupus erythematosus (SLE)
- Solid organ transplant recipients
- Tobacco usage
Screening
NHS cervical screening programme established in 1988 has been highly successful in reducing the incidence of the cervical cancer.
Who is eligible?
All eligible women aged between 25 and 64 in England are automatically invited for the cervical cancer screening test. Women aged 25 to 49 receive invitations every 3 years. Women aged 50 to 64 receive invitations every 5 years.
How does cervical screening work?
The test is designed to detect precancerous cells with a aim to treat pre-cancer so they do not progress to cancer. Approximately 1 in 20 women have abnormal cell on smear. In addition to the abnormal cells, test for human papillomavirus (HPV) is carried out in those with low-grade cell abnormality. HPV test helps to decide if woman needs to be referred to colposcopy clinic.
Many women with abnormal tests are referred to the colposcopy clinic for further tests. Remaining low-risk women with abnormal test are advised to have earlier than normal repeat of their screening test.
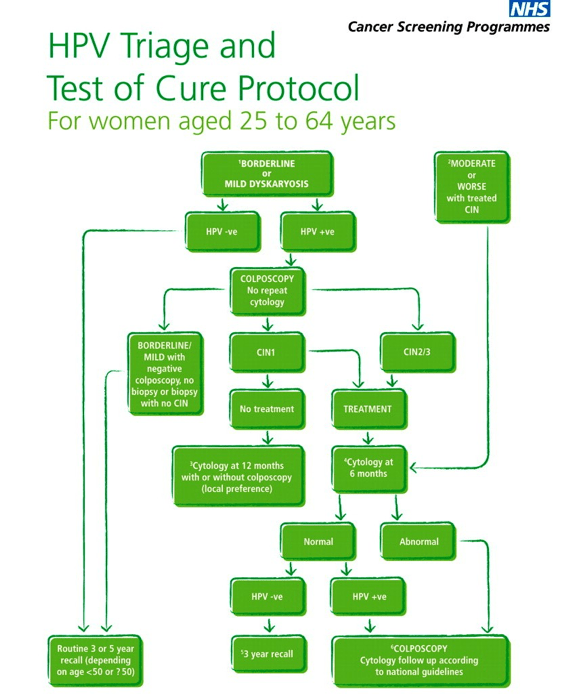
What happens at colposcopy clinics?
At colposcopy clinic women are examined using colposcope and acetic acid and Lugol’s iodine solution. A small punch biopsy may be taken to determine if high-grade pre-cancer changes (CIN 2 or higher) are present. If found, loop biopsy treatment is performed under local anaesthetic.
After treatment with loop biopsy women are advised to have a test-of-cure screening test in six months at GP. This test is examined again for abnormal cells and presence of HPV virus. If found negative women are discharged back to routine three or five yearly screening test.
Is my risk of cancer higher if I do not take part in screening?
Not taking part in cervical screening programme is the biggest risk for developing cervical cancer. However it is also important to note that cervical screening is not 100% accurate and do not prevent all cervical cancers.
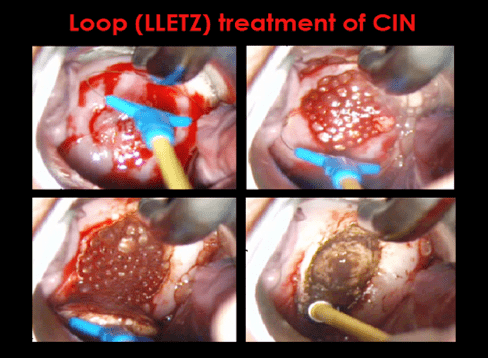
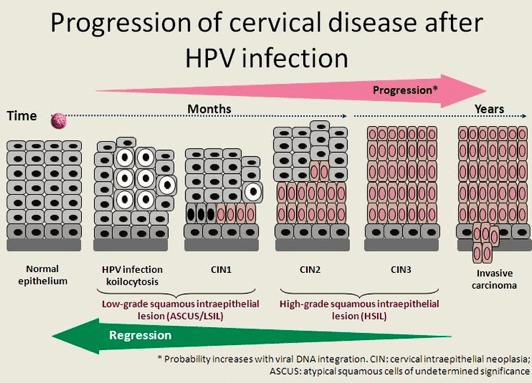
Cervical Pre-cancer – Cervical Intra-epithelial Neoplasia (CIN)
- 30,000 new cases per year in UK
- 94 cases per 100,000 women per year in UK
- Life-time risk of 1 in 25
- Peak incidence of CIN is in 25-29 age group
- Screening age in England is 25-65
- Incidence rates have increased significantly since 1970s
Symptoms
- Irregular vaginal bleeding
- Bleeding in-between periods
- Post-coital bleeding
- Post-menopausal bleeding
- Chronic vaginal discharge
- Painful sex
- Weight loss
- Bladder or bowel problems
- Leg swellings
- Bone pain
Investigations
Diagnosis
Speculum examination and Colposcopy
Punch or Loop (LLETZ) biopsy of the cervix
Staging
Examination under anaesthetic with cystoscopy (camera check of inside of bladder) and rectal examination if tumour extension to bladder or back-passage is suspected
CT scan, MRI or PET scan to check for cancer spread to lymph nodes and other parts of body
FIGO Stage
- Stage 1 – Cancer is limited to the cervix, Microscopic (A) or Visible with eye (B)
- Stage 2 – Cancer has spread to nearby parts such as the upper vagina (A) or to the side of cervix (B)
- Stage 3 – Cancer has spread to lower vagina (A) or to pelvic side wall or dilated ureters (urine tubes connecting kidney to bladder) (B)
- Stage 4 – Cancer has spread to bladder, back-passage (A) or other parts of the body (e.g. Lungs) (B)
Treatment
Surgery
Surgery is the main treatment for cervical cancer up to FIGO Stage 1B.
For very small microscopic cervical cancer (FIGO Stage 1A1) treatment with loop is sufficient.
For FIGO Stage 1A2 and small size 1B1, loop treatment (if fertility is required) or simple hysterectomy (if family complete) along with pelvic lymph node removal is recommended.
For FIGO Stage 1B1 large size and selected cases of Stage 1B2, radical trachelectomy (if fertility is required) or radical hysterectomy (if family complete) along with pelvic lymph node removal is recommended.
Radiotherapy and Chemotherapy
FIGO Stage 2A and higher are usually treated with radical chemo-radiotherapy combination. Chemotherapy improves efficacy of radiotherapy on cervical cancer.
Some selected cases of FIGO Stage 1B2 (where need of radiotherapy is anticipated to be higher regardless of outcome of operation) may also be recommended to have treatment with radical chemo-radiotherapy combination.
After operation if lymph nodes are found positive or if margins of radical hysterectomy are involved, further treatment with chemo-radiotherapy may be required.
Women who are not fit for surgery or those with advanced stage of cancer may also be treated radiotherapy, chemotherapy or combination.
Survival
Survival in cervical cancer mainly depends on the
- FIGO stage of the cancer (how far has cancer spread)
- Grade of cancer (how aggressive cancer cell look under microscope)
- Morphology (cell type) of cancer
- Age
- Fitness of woman
In general at least 67 out of 100 women with cervical cancer will live 5 years or more.
- Stage 1 – 95 out of 100 will survive 5 years or more (95%)
- Stage 2 – 50 out of 100 will survive 5 years or more (50%)
- Stage 3 – 40 out of 100 will survive 5 years or more (40%)
- Stage 4 – 5 out of 100 will survive 5 years or more (5%)
Survival has improved continuously over last 4 decades.
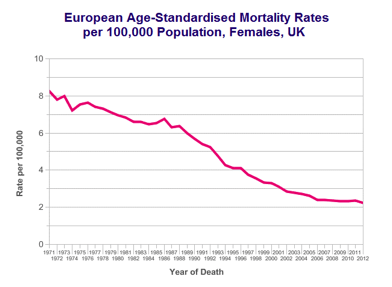
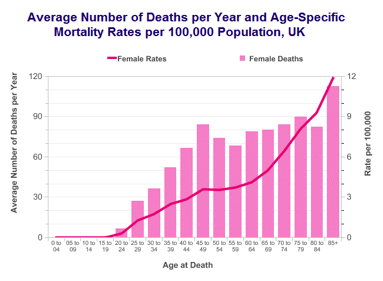
Further Information and Support
https://www.macmillan.org.uk/search/search.html?query=cervical+cancer


